 |
Centre for Australian National Biodiversity
Research
|
Supervisor: Bob Godfree
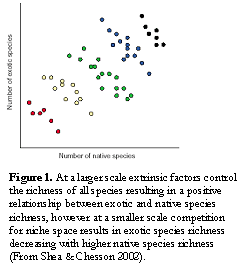
The susceptibility of a vegetation community to invasion is thought to be to a certain extent influenced by the community’s richness (Elton 1958, Naeem et. al. 2000). Indeed many experimental studies have demonstrated a negative relationship between community richness and the richness of exotic species (e.g. Naeem et. al. 2000). One explanation for this is that high native species richness leads to more resources being utilised, leaving fewer resources and niche opportunities for invaders (Shea & Chesson 2002, van Ruijven et. al. 2003). However large-scale observational studies often demonstrate the opposite relationship (e.g. Foster et. al. 2002). These seemingly conflicting results have been explained by the presence of confounding extrinsic factors that are controlled in experimental studies and models (Levine 2000, Shea & Chesson 2002). Shea & Chesson (2002) reconciled these findings by suggesting that the scale of the study will affect the relationship (Figure 1). At a large scale where extrinsic factors vary, a positive relationship may be found, as both native and exotic species richness respond to these factors in the same way. However at a smaller scale where these factors are likely to be more uniform, the number of exotic species is instead reduced by native species richness.
The aim of the study was to investigate patterns of weed invasion in subalpine vegetation both at a large and a small scale, and thus determine whether the richness model of Shea & Chesson (2002) holds true, particularly as the idea that negative relationship even occurs in naturally is highly contentious (Wardle, 2001). We also examined relationships between exotic and native species in terms of biomass, an often neglected means of describing community characteristics.
 The
second aim of this study was to investigate the distribution and abundance of
Trifolium repens, or white clover (Figure 2). T. repens was selected
because it is a known invader in subalpine vegetation, and additionally because
there are environmental risks associated with the potential release of a genetically
modified, virus-resistant T. repens (Godfree et. al. in press).
The
second aim of this study was to investigate the distribution and abundance of
Trifolium repens, or white clover (Figure 2). T. repens was selected
because it is a known invader in subalpine vegetation, and additionally because
there are environmental risks associated with the potential release of a genetically
modified, virus-resistant T. repens (Godfree et. al. in press).
The study area, Grassy Creek, is located in Namadgi National Park approximately 65 km south of Canberra, ACT. Grassy Creek lies at 1200 to 1300 metres a.s.l. and is classified as subalpine.
Eight sites were selected from a larger pool of randomly selected sites. These 8 sites were selected to be most representative of the various community types in terms of species composition. Four of the sites were from woodland communities and 4 were from grassland communities.
At each site a 1 m x 1 m quadrat (plot) was laid out, divided into 25 subplots of 20 cm x 20 cm. All above ground biomass (except for large Eucalyptus species) was clipped from each subplot and bagged. Individual species from each subplot were subsequently separated and identified.
All plant material was dried at 95 degrees C for three days until constant weight was achieved and then weighed.
Grassland communities were dominated by native perennial grasses, such as Poa sp. and Themeda triandra (kangaroo grass). Geranium antrorsum was the most abundant herb. Trifolium repens was the most abundant exotic herb across all grassland sites, although two species of exotic grasses, Poa pratensis (Kentucky bluegrass) and Holcus lanatus (Yorkshire fog) were found in higher abundances.
 Across
all woodland sites Poa spp. were most abundant. Woodland sites had a
shrubby understory consisting of species such as the common Fabaceous shrub
Bossiaea foliosa. The overstory was dominated by Eucalyptus stellulata
(black sallee). T. repens was relegated to second-most abundant exotic
herb in the woodland community behind the Asteracean species Hypochoeris
radicata (flatweed), although Holcus lanatus was the most abundant
exotic species (and third most abundant species overall in the woodlands).
Across
all woodland sites Poa spp. were most abundant. Woodland sites had a
shrubby understory consisting of species such as the common Fabaceous shrub
Bossiaea foliosa. The overstory was dominated by Eucalyptus stellulata
(black sallee). T. repens was relegated to second-most abundant exotic
herb in the woodland community behind the Asteracean species Hypochoeris
radicata (flatweed), although Holcus lanatus was the most abundant
exotic species (and third most abundant species overall in the woodlands).
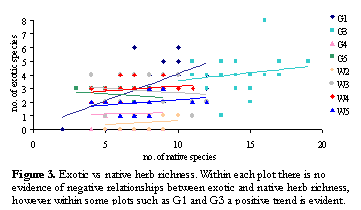
At the large scale (across all plots) exotic species richness increased with native species richness as expected. However when this relationship was examined at the small scale (within each plot) there was no evidence of the predicted negative relationship, indeed a positive relationship was evident within some plots particularly in G1 (Figure 3). These relationships were due to the strong positive relationship between native and exotic herbs, the most species-rich functional group in the study area.
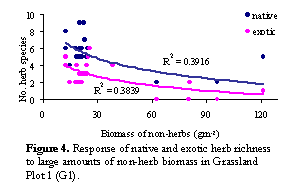 The
positive relationship between exotic and native herb richness in G1, appears
to be related to the observation that both native and exotic herbs decline with
increased non-herb biomass (Figure 4). The bulk of non-herb biomass at this
high level was due to the presence of a single species not found at any other
site, Poa labillardierei (river tussock), a large native tussock grass.
This suggests that competition is occurring at a small scale, however is driven
by the biomass of a single species, rather than resources being consumed by
high richness.
The
positive relationship between exotic and native herb richness in G1, appears
to be related to the observation that both native and exotic herbs decline with
increased non-herb biomass (Figure 4). The bulk of non-herb biomass at this
high level was due to the presence of a single species not found at any other
site, Poa labillardierei (river tussock), a large native tussock grass.
This suggests that competition is occurring at a small scale, however is driven
by the biomass of a single species, rather than resources being consumed by
high richness.
Unlike richness relationships, the biomass of native and exotic species were not related at the large scale. However, at the smaller scale, a negative relationship was found in three out of eight plots. Within these plots, it appeared that exotic biomass declined with increasing abundance of the dominant species, such as P. labillardierei, or the dominant functional group, primarily grasses, suggesting competition by a single dominant group/species rather than by high numbers of native species.
|
Figure 5. Synthesis of results of study. Each plot is ranked in order of increasing biomass of exotics, and divided into three groups (a) low exotic biomass (b) moderate exotic biomass; and (c) high exotic biomass. Values in the table are averaged across plots in each group. |
||||||||||||||||||||||||
|
We also found that plots containing low amounts of exotic biomass tended to have higher species richness, whereas plots with high amounts of exotic biomass contained few exotic species (Figure 5). It is clear that these two parameters yield different results – many exotic species does not necessarily mean that the biomass of exotics is high. In addition, it appears that where exotic biomass is low (but species-rich) the majority of species are annual/biennual herbs, whereas where exotic biomass is high (but species-poor), all are perennial and the majority are grasses (Figure 5).
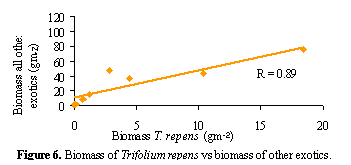
T. repens was found to co-invade with other exotic species
(Figure 6), indicating that T. repens is unlikely to be found in high
abundances on its own; it occurs where other exotics also perform well. The
relationship between T. repens and native grasses was also examined.
In only one instance was there evidence that T. repens was limited by
grass biomass, possibly because only once did grass biomass reach this magnitude
(driven by the presence of Poa labillaridierei). This indicates that
T. repens will not be limited by native grass biomass unless it is very
high, which is rare in the communities studied and requires the presence of
large tussock grass species such as P. labillardierei.
Measurements of biomass and richness are two different ways of describing a community and do not necessarily follow the same relationships. Our data suggests that when investigating invasions we must ask whether it is the richness or biomass of exotics that has the greatest effect on communities, since models such as that postulated by Shea & Chesson (2002) do not take into the biomass of exotic species. In reality, however, many invaded vegetation communities are overrun by a dominant, vigorous exotic invader.
At the small scale there appears to be a competitive process whereby a single dominant species, or a dominant functional group is able to limit other species. Therefore at the small scale, relationships in subalpine vegetation do not seem to be driven by richness but by large amounts of biomass, or dominance of one group. These relationships require more investigation, and may need to be incorporated into invasion theories.
Thanks to Bob Godfree for his encouragement, advice, and enthusiasm throughout the project, and for showing me just how interesting and fun the study of ecology can be. Much appreciation is extended to Brendan Lepschi and Dave Mallinson for their efforts in identifying many of the species, particularly grasses. Also thanks to Matt Woods for his help in the field, particularly amongst the heat and the flies! Finally I thank CSIRO and the CPBR for providing me with the chance to experience this valuable opportunity.
Elton, C.S. (1958) The ecology of invasions by animals and plants. Methuan & Co. Ltd, London.
Foster, B.L., Smith, V.H., Dickson, T.L. & Hildbrand, T. (2002) Invasibility and compositional stability in a grassland community: relationships to diversity and extrinsic factors. OIKOS, 99: 300-307.
Godfree, R., Chu, P.W.G. & Woods, M.J. (in press) White Clover (Trifolium repens) and associated viruses in the subalpine region of southeastern Australia: implications for GMO risk assessment.
Levine, J.M. (2000) Species diversity and biological invasions: relating local processes to community pattern. Science, 288: 252-254.
Naeem, S., Knops, J.M.H., Tilman, D., Howe, K.M., Kennedy, T. & Gale, S. (2002) Plant diversity increased resistance to invasion in the absence of covarying extrinsic factors. OIKOS, 91: 97-108.
Shea, K. & Chesson, P. (2002) Community ecology theory as a framework for biological invasions. Trends in Ecology and Evolution, 17: 170-176.
van Ruijven, J., De Deyn, G.B. & Berendse, F. (2003) Diversity reduces invasibility in experimental plant communities: the role of plant species. Ecology Letters, 6: 910-918
Wardle, D.A. (2001) Experimental demonstration that plant diversity reduces invisibility – evidence of a biological mechanism or a consequence of sampling effect? OIKOS, 95: 161-170.